Life Sciences Research
Insights with Impact
Our Solutions
-
 solution
solution
Therapeutics, Prophylaxis and Diagnostics
Battelle operates the world’s largest non-governmental containment laboratories that help clients deliver life-saving diagnostic and therapeutic solutions for victims of chemical and biological warfare or pandemic diseases. -
 solution
solution
Precision Public Health
Battelle delivers the right intervention, to the right population, at the right time. Our interdisciplinary team of public health experts, data scientists and molecular geneticists improve health outcomes while promoting health equity. -
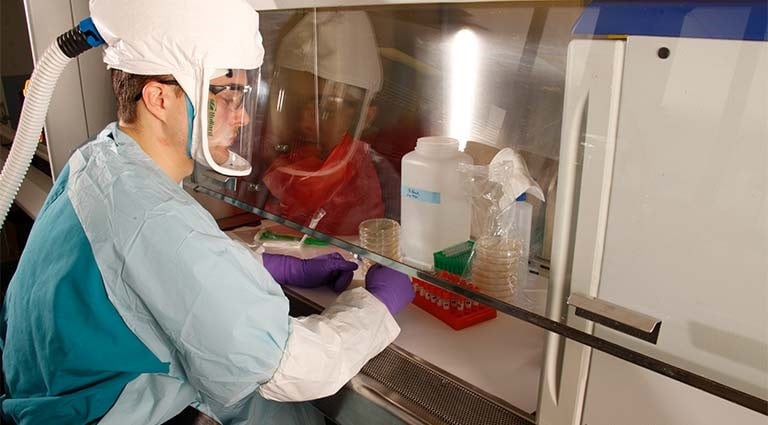 solution
solution
Chemical Agent Countermeasures
Battelle is a global leader in understanding the impact of chemical agents on our national security. Our experts execute the most thorough testing procedures and processes available under stringent quality management systems, providing guiding insights for the most important decisions. -
 solution
solution
Biosecurity and Pandemic Preparedness
Our Biomedical Research Center conducts secure, state-of-the-art research, development, testing, and evaluation to enable proactive biosecurity preparedness critical to our national security, economic strength, and the well-being of people.
By the Numbers
40 +
Years of Experience
200 k+
ft2 biological research laboratories
77 k
ft2 of chemical research laboratories
22 k+
ft2 of BSL-3 lab space
70 k+
ft2 of BSL-2 lab space
World Class Facilities
Our highly specialized laboratories and equipment enhance the quality and value of our work for faster, more reliable results. Our facilities include the largest privately owned enhanced Biosafety Level 3 (BSL-3+) facility in the United States as well as 5 chemical surety laboratories.
Our Experts
-
Nicole Brennan
Public Health Thought Leader and Division ManagerNicole brings over 20 years of public health and healthcare quality expertise to Battelle’s Health Research Division. She leads a team of domestic and international health researchers, scientists and practitioners with expertise in diverse aspects of health science, including healthcare quality measurement, environmental health, genomics, gerontology, behavioral science, preparedness, and military health. Nicole and the members of her team enable discovery to decision support through applied science. -
Gabe Meister
Research LeaderGabe Meister is a Sr. Scientist with 20 years of experience working in the discovery and preclinical phases of vaccine and drug development. Dr. Meister has led multiple programs at Battelle that have resulted in FDA approval of novel therapeutics for the treatment of rare and life-threatening diseases. Most recently Dr. Meister leads a diverse group of scientists and engineers in developing novel drug delivery technologies. -
Drew Cawthon
Business Line Director, Life Sciences ResearchDrew manages just over 300 staff engaged in pre-clinical and clinical research for both government and commercial clients. Drew’s team provides integrated scientific and technological capabilities in high hazard and biocontainment toxicology, assay development and validation services, and toxicology and pharmacology for support for investigational new drug studies. Drew and the members of his team also support clinical research through the Battelle Public Health Research Center in Baltimore, Maryland and provide onsite scientific support for the Centers for Disease Control and Prevention in Atlanta, GA. -
Daniel Sanford
Research Leader, High-Containment BiologyDr. Daniel Sanford is a Research Leader at Battelle’s Biomedical Research Center (BRC). He has served as Study Director on more than 75 studies conducted in Battelle’s high-containment laboratory during the past 16 years, with a focus on model development, bacterial/viral pathogenesis, and medical countermeasure development/evaluation. Dr. Sanford has experience with a number of pathogens/toxins, including Bacillus anthracis, Yersinia pestis, Burkholderia pseudomallei, Burkholderia mallei, Pseudomonas aeruginosa, botulinum neurotoxin, ricin, Zika Virus, rabbitpox virus, and SARS-CoV-2. Dr. Sanford played a significant role in overseeing the execution of studies to support the FDA licensure of Raxibacumab, Anthim, and Tembexa. He has participated in four FDA directed inspections of the BRC, which involved FDA review of nine studies for which he served as Study Director. In addition, Dr. Sanford has presented at FDA on his experience serving as a Study Director on GLP studies conducted in a high-containment lab.